Our Mission
«metaLead revolutionizes the way we treat toxic metals, starting with the most dangerous one - lead»
Lead poisoning remains an unmet medical need.
Lead (Pb) is a nonessential, toxic metal widely spread in the environment and affects vast populations worldwide. No blood Pb level (BLL) is deemed safe, and Pb at even very low concentrations shows destructive effects on various body organs. Nevertheless, health authorities determine threshold values from which environmental and medicinal interventions should occur. Most countries, including Switzerland and the EU, adopt the WHO recommendation of 5 µg/dL as this value. In the USA, the CDC recently reduced this value to 3.5 µg/dL, acknowledging the devastating influence of Pb at even such levels.
Based on these values, 16% of the US children, every third child globally, and every second adult in the USA are poisoned with Pb. While at highly acute poisoning, Pb kills (almost 1 M annually worldwide), lower poisoning levels cause various symptoms and diseases, mainly kidney impairment, anemia, high blood pressure, and other cardiovascular failures, fertility issues for both genders, and most critically, cognitive and neurological damages. The brain and the central nervous system are susceptible to Pb poisoning since Pb(II) ions resemble calcium ions (Ca(II)), which are highly essential in these organs. As a result, Pb, as a neurotoxicant, easily crosses the blood-brain barrier, resulting in amplified damage to the brain. This includes a proven inhibition of cognitive development, reduction in IQ (which is on its own quantified as a loss of 0.33% of the GDP of the USA, equal to $52 billion and 0.31% of the GDP in the EU, equivalent to $55 billion), mental and behavioral problems, such as ADHD, etc.
Pb exposure is caused by ingestion of contaminated food and water and inhalation of Pb particles. Even though the massive industrial use of Pb in developed countries was prohibited years ago, the long-lasting effect is still apparent. It results from mainly, but not only, the following sources:
- Pb-based paints that were banned in North America in the late 1970s and by the EU in 2003 still exist in about 30 million houses in the USA. The CDC, the Agency for Toxic Substances and Disease Registry (ATSDR), and other relevant agencies declare that every house built before banning Pb-paints is unsafe for children below 6 years old. The only solution in these cases is a complete renovation of the houses, which is estimated to cost over $500 billion.
- Household water is still supplied in many countries, including North America, with Pb pipes and service lines. In a report from March 2021 by the White House, over 6 million houses and more than 400,000 schools in the USA receive their water contaminated with Pb. A replacement of these lines only in the USA is estimated to cost $45 billion.
- Pb-additives in gasoline that were banned at the beginning of the 2000s are accumulated in the environment, as reflected in many reports, for example, the latest topsoil survey by the EU that detected Pb contaminants all over Europe.
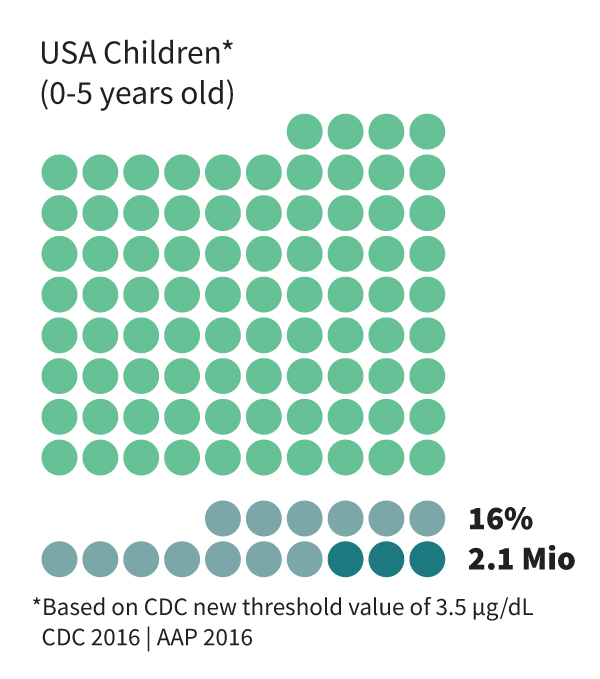
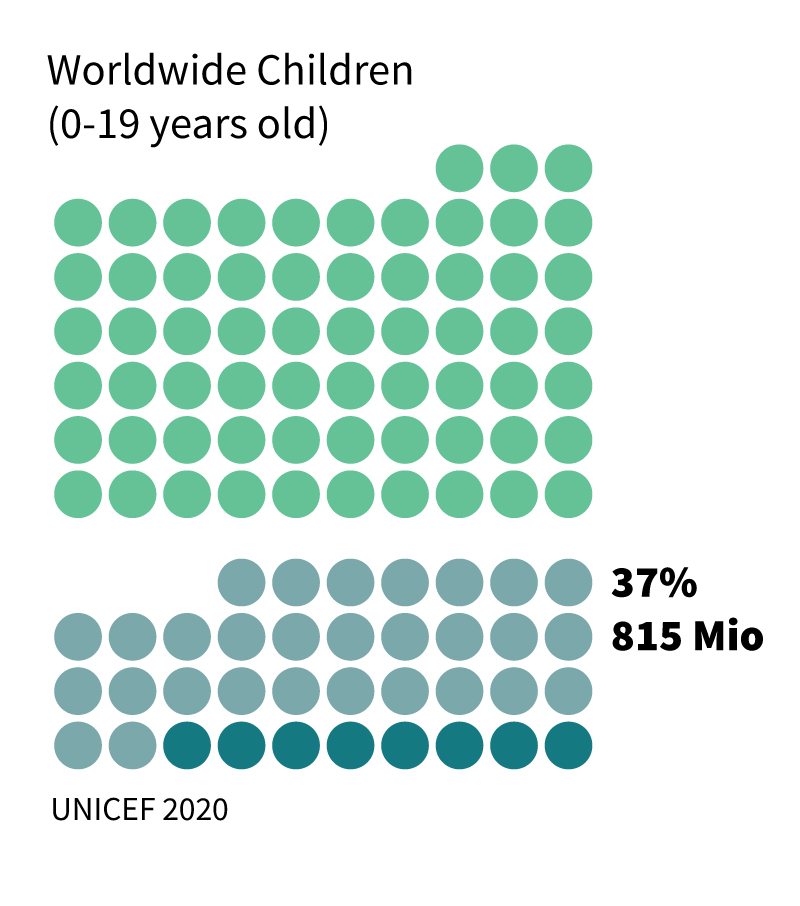
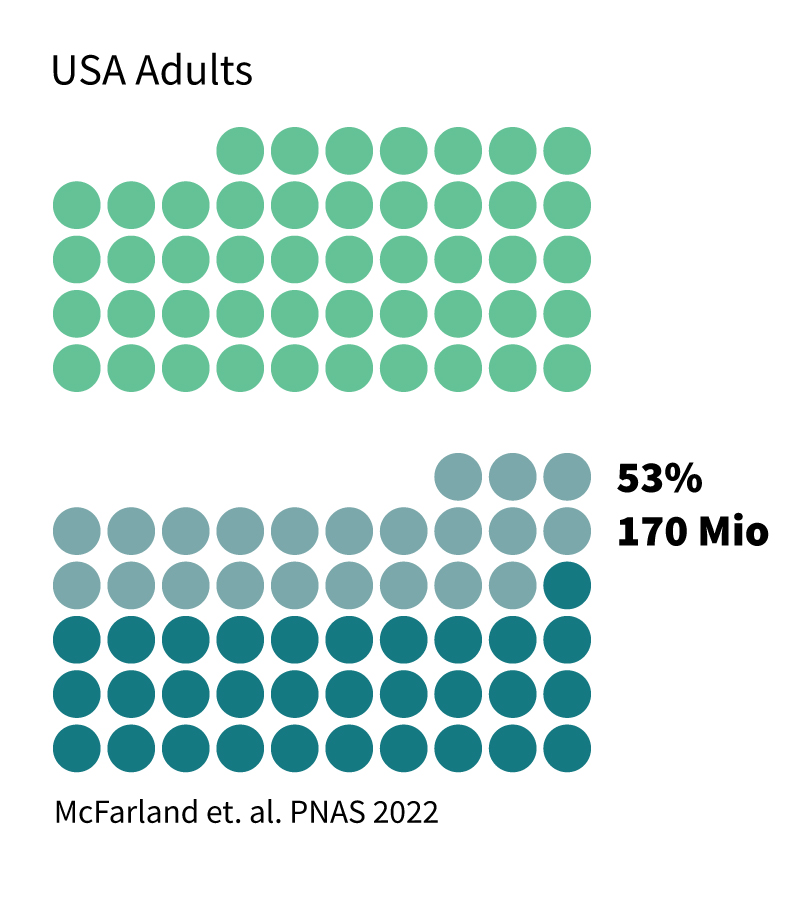

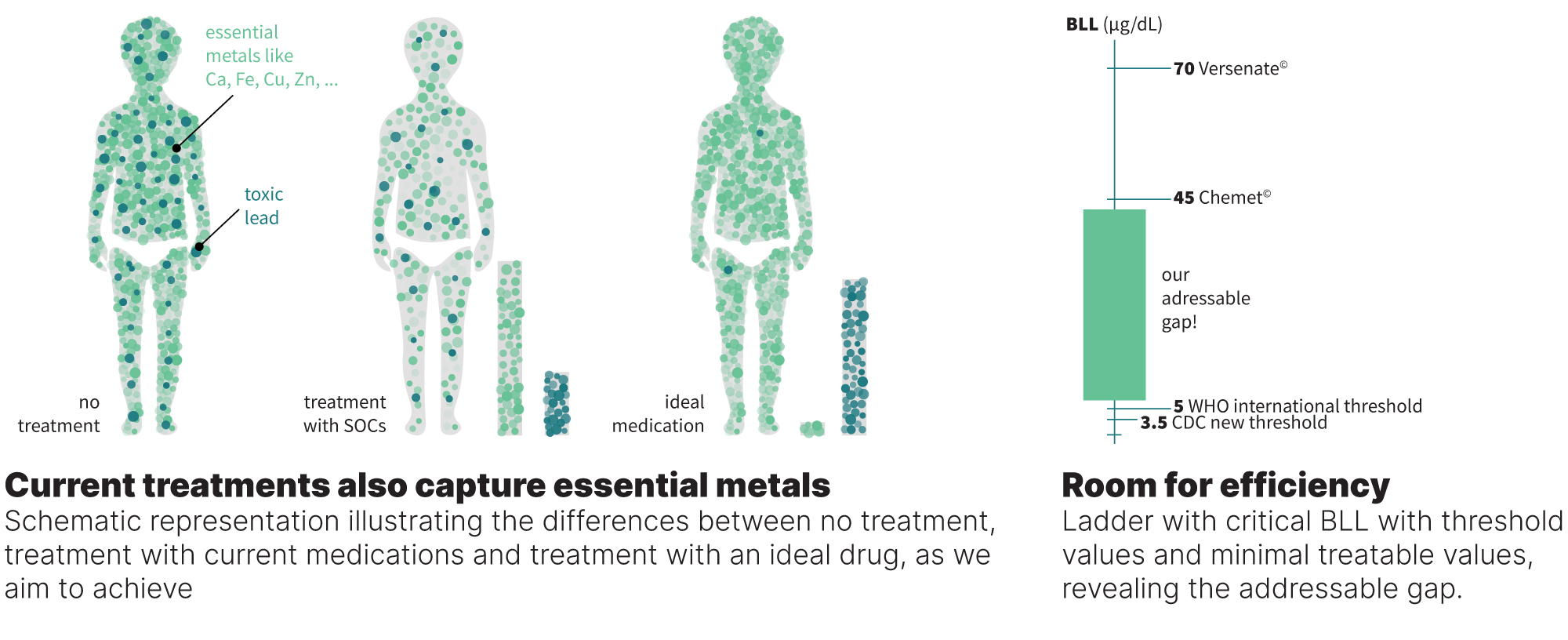
Current therapies do not meet the threshold requirements.
The FDA and other agencies approved five small molecules as chelating agents to treat Pb poisoning. Three of the five compounds are no longer used due to severe side effects and poor performance. Only two drugs are still in practice: Chemet (succimer; dimercaptosuccinic acid; administered orally) and Versenate (calcium disodium EDTA; administered intravenously). These drugs maintain some ability to clear Pb(II) ions but suffer from several disadvantages, most critically:
-
- Their binding affinity to Pb(II) ions is not as high as to other essential metal ions such as Ca(II), Fe(III), and Zn(II), which results in poor metal selectivity. This causes the depletion of essential metal ions upon treatment and the requirement to increase the dosage, which also causes off-target effects, including anemia, headache, vomiting, nausea, muscle and joint pain, fever, diarrhea, and more.
- Even at acute Pb levels, the drugs struggle to reduce BLL and Pb in the brain over time, hence multiple cycles of treatment are required.
Due to the above, the two drugs are given only in severe cases of Pb poisoning: Chemet is administered at BLL of at least 45 µg/dL and Versenate at values higher than 70 µg/dL. These values are 12 and 20 times higher than the reference value of the CDC, respectively, and 9 and 14 times higher than the WHO one.
People of all ages who suffer from Pb poisoning of up to 45 µg/dL cannot be treated medicinally, leaving them without an effective solution against Pb. Children and adults diagnosed with elevated BLL that cannot be medicinally treated undergo a risk assessment by allocated clinics in major hospitals. This includes tight monitoring of BLL, neurological, phycological, and cognitive evaluations and identifying and removing Pb sources when possible. The latter enables controlling the exposure levels and avoiding further consumption of Pb. However, Pb cannot be removed from the body without medicinal treatment. This unmet medical need of millions of people who currently cannot expel Pb from their bodies is at the forefront of our goals.
Our value proposition
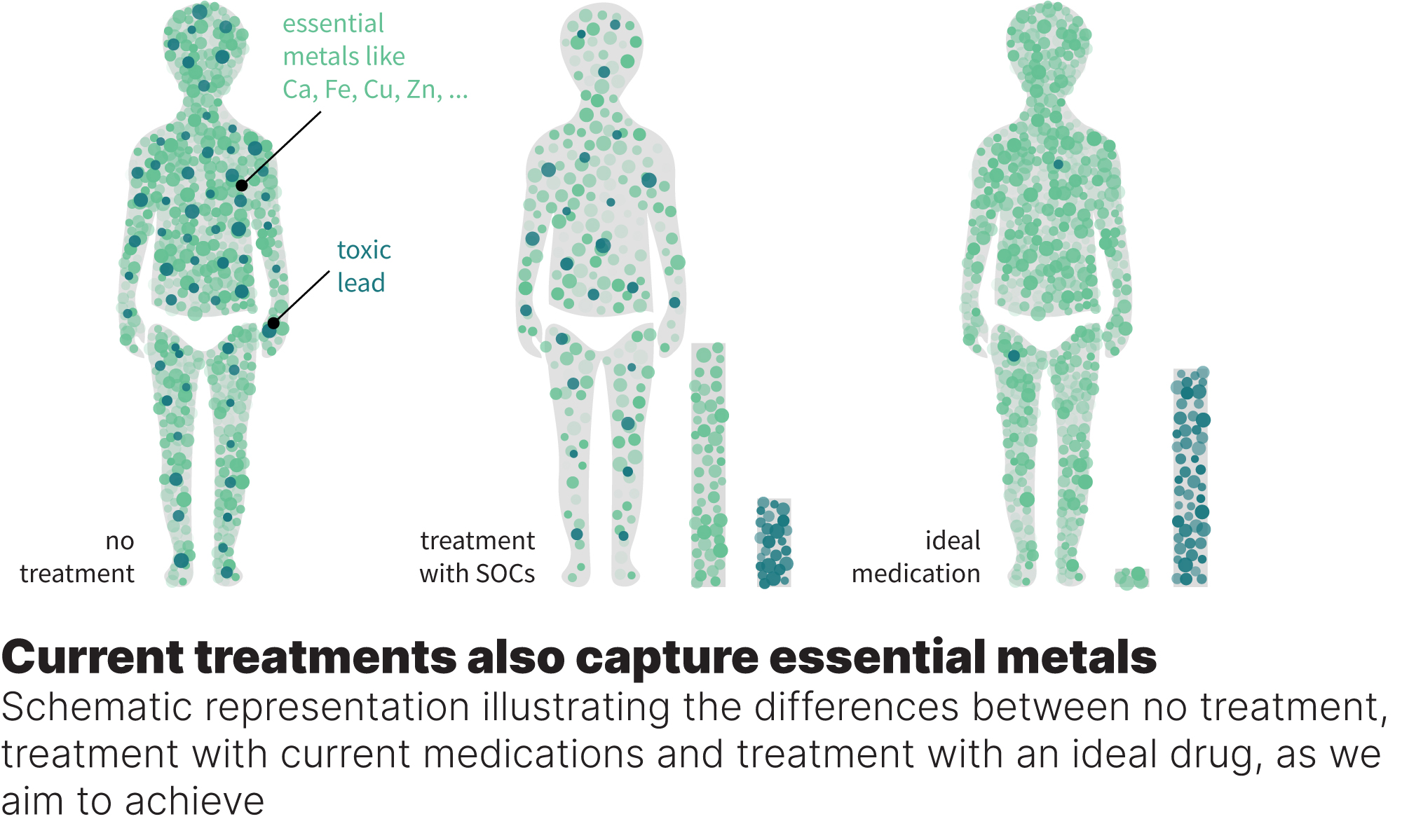
Our drug candidates will help children and adults effectively remove toxic Pb from their brains and whole bodies. The lead candidate achieves a 4 times higher efficacy without the risk of eliminating essential metals like Zn and Ca compared to the currently available treatment. This is a game-changing perspective in fighting Pb poisoning.
Our peptides outperform the SOCs in recovering Pb-poisoned human cells.
A family of novel short peptides was designed for our goals in selective metal detoxification. Of 52 peptides that were synthesized, eight exhibited excellent abilities to recover Pb-poisoned human cells and outperformed the two SOCs by up to 4-folds. The results of the most successful three candidates are shown in Figure 5. These candidates, which also exhibited no cellular toxicity, were investigated for relevant chemical and physical properties, including their chemical and enzymatic stability, solubility, and Pb binding affinity and selectivity with respect to biologically relevant essential metal ions (Ca(II) and Zn(II)) by various analytical tools (UV-Vis, HR-ESI-MS and ITC). These examinations revealed the high affinity to Pb(II) ions and significant Pb selectivity of three candidates among the eight as they did not bind Zn(II) or Ca(II) ions, even when present at 10- or 100-fold excess, respectively. In addition, their high solubility and stability in human blood serum revealed no undesired degradation or oxidation in 48 hours.
A selected PoC candidate, MTL-01, outcompetes the SOCs in efficacy and selectivity (no elimination of essential metals that cause off-target effects) while showing high blood stability and no signs of toxicity.
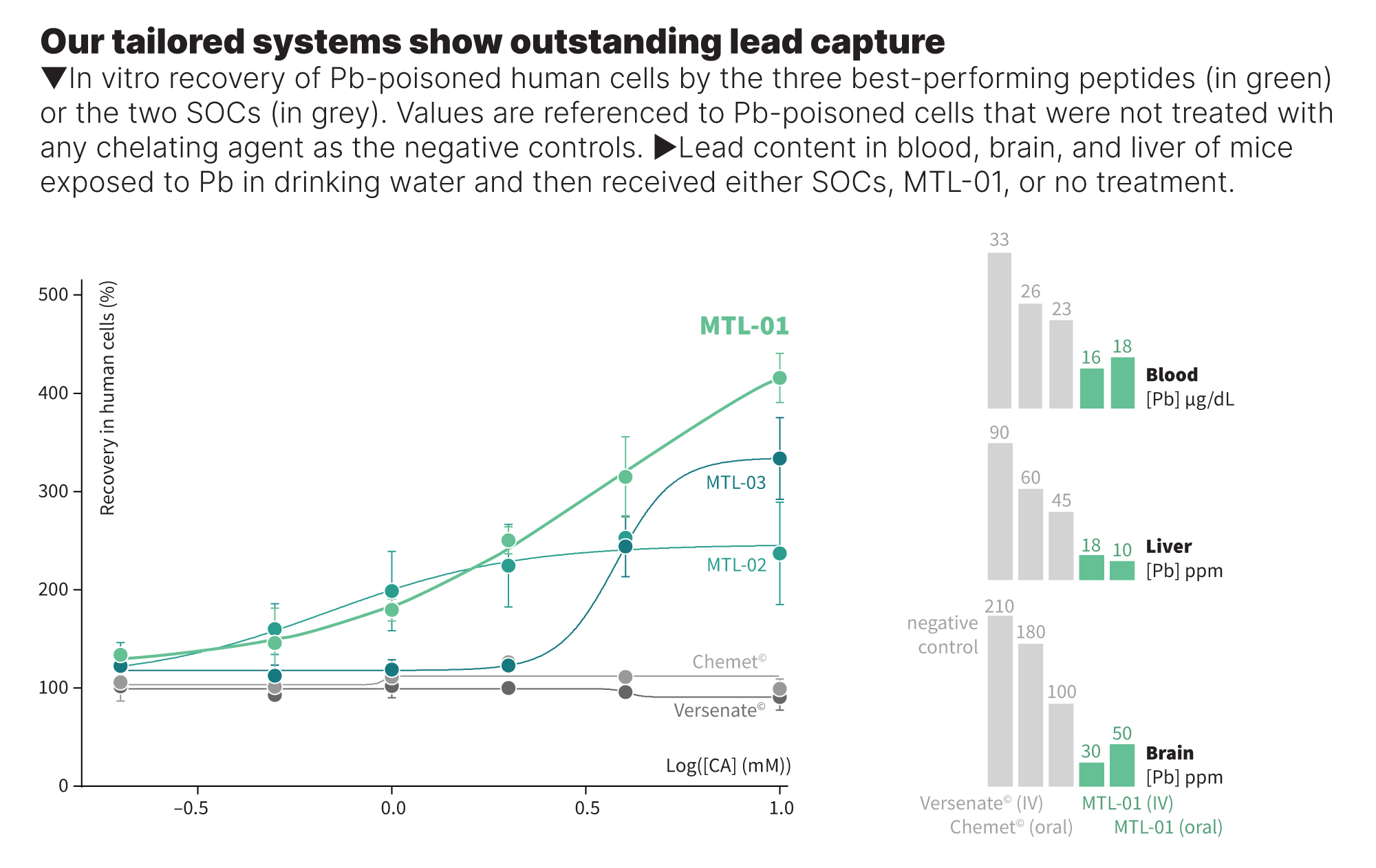
We tested MTL-01 as the PoC candidate in mice upon both oral (gavage) and IV administrations to determine its efficacy, stability, and safety. The BLL and the Pb content in the brain and liver of mice receiving MTL-01 were significantly reduced compared with untreated mice or animals treated with the two SOCs. While the Pb in blood was reduced by 30-60% compared with the SOCs, Pb in the internal organs was decreased by 90-500%. These data are aligned with published reports on the SOCs and corroborate the suspension that Versenate causes Pb accumulation in the brain.
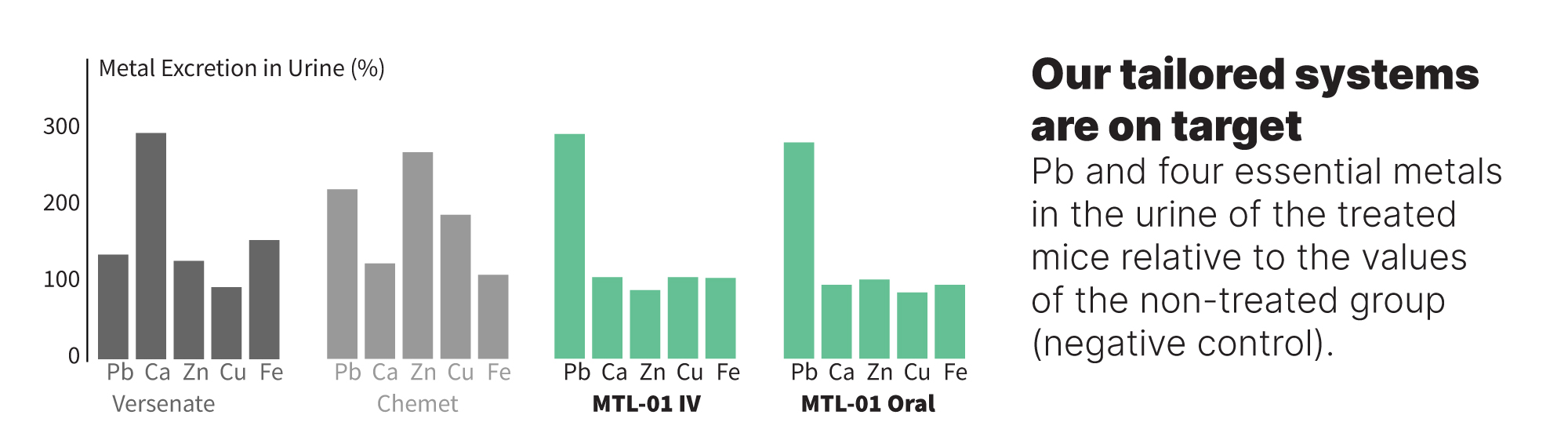
Furthermore, metal analysis in the urine reveals that not only does MTL-01 expels only Pb without changing the concentrations of the essential metals, but the lead compound also depletes 30-115% more Pb than the SOCs. Noteworthy, elevated urinary Pb in the groups treated with MTL-01 implies that the candidate acts as a chelating agent while expelling the toxic metal through the urinary system.
